Hydrogen Education
Hydrogen education is a core part of Horizon Fuel Cell Shop’s mission. We’ve been part of the Horizon Fuel Cell Europe Group since our founding, along with two sister organizations dedicated to education, Horizon Educational and Heliocentris Academia. And we’re proud that our fuel cells are used in education competitions worldwide, including the Shell eco-marathon and Monaco Energy Boat Challenge.
Discover more about the exciting education uses our fuel cells have been put to in our blog.
Hydrogen 101
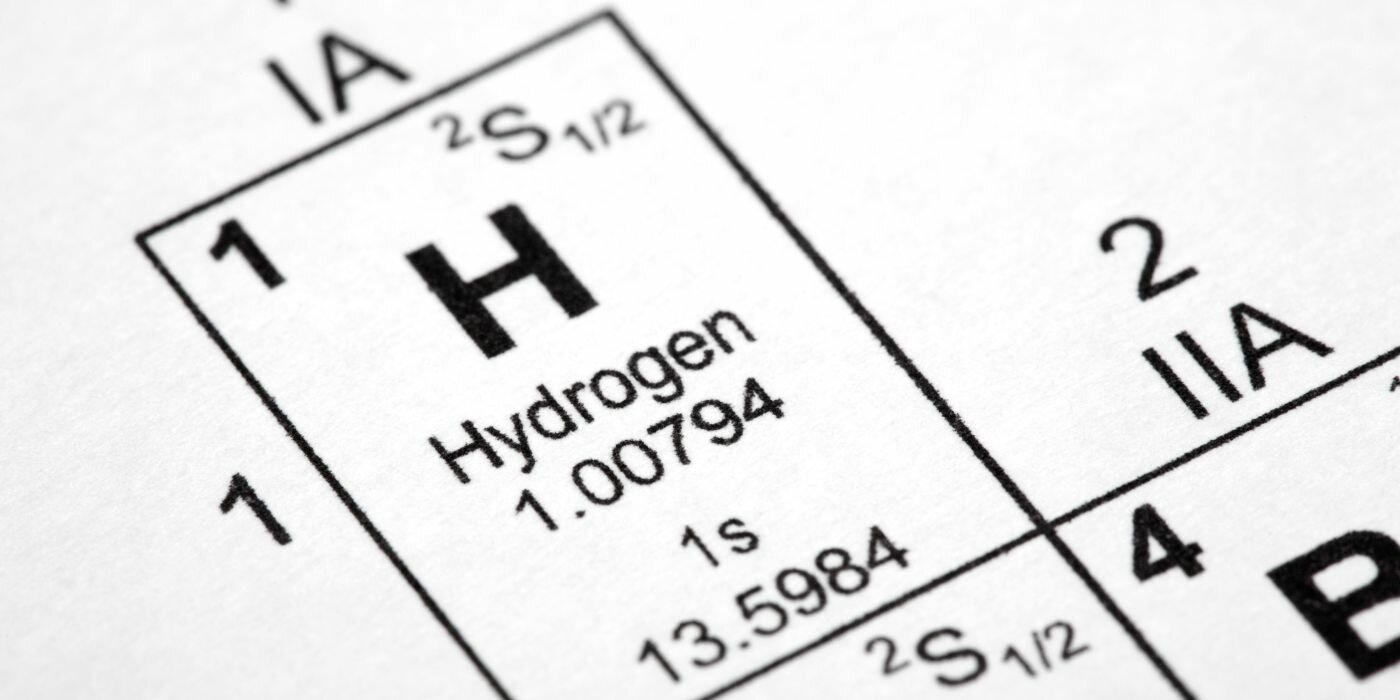
What is hydrogen? How is it different from other chemical elements?
Hydrogen (H) is the lightest element in the universe, with an atomic number of 1. It’s extremely reactive, meaning that vast majority hydrogen on earth is found in molecular forms like water (H2O) or other organic compounds like methane (CH4) and Heptyne (C7H12).
In fact, hydrogen consists of around 75% of all matter in the universe. Stars such as the Sun are mostly composed of hydrogen in a plasma state.
At standard atmospheric conditions, hydrogen is a gas composed of two diatomic molecules (H2).
How is hydrogen used?
Hydrogen is a medium for storing and transporting energy. This means that, like all fuels (such as gasoline or natural gas) it’s volatile and easily combustible. But the main difference between hydrogen and other fuels is that when it’s burned, the only emissions are water and heat.
This is why hydrogen’s is seen as one of the key components in the clean energy transition. Other benefits of hydrogen include:


- Existing infrastructure: hydrogen can easily be blended into existing natural gas infrastructure. Some newer domestic boilers can even run on a blend of natural gas and hydrogen. This means massive cost savings compared to building new pipes and encouraging consumers to adopt new equipment.
- Storing renewable energy: It’s becoming difficult for industries to find places to store all the renewable energy produced from wind farms and solar energy. When the sun’s out and wind is blowing this isn’t a problem, but when these sources of energy can’t keep up with demand (on a dark evening or windless day), storing their energy becomes essential. This can be done in stationary fuel cells, cryogenic liquid, or loosely bonded hydride compounds for long-term storage. Hydrogen can also be stored in natural geological formations like salt caves or unmineable coal areas.
- Energy density: hydrogen has a much higher energy density than greenhouse gas. The storage density of hydrogen of hydrogen is actually 120 MJ/kg, almost three times more than diesel or gasoline. This means more energy can be produced from less gas. In fact, when used as part of a fuel cell, 1 kg of hydrogen can produce 33 kWh of electrical energy. Natural gas can only produce around 13 kWh/kg.
How is hydrogen produced?
Hydrogen can be produced many different ways. The most common are steam-methane reforming and electrolysis (splitting water with electricity).
Steam-methane reforming is when methane from natural gas is heated with high-temperature steam (700°C–1,000°C) and a catalyst to produce hydrogen. During this process methane reacts with steam and a catalyst (usually nickel-based) under 4-25 bar pressure to produce hydrogen, carbon monoxide, and a small amount of carbon dioxide.

Steam-methane reforming reaction:
CH4 + H2O (+ heat) → CO + 3H2
Hydrogen can also be produced via electrolysis. This involves passing electricity through water, splitting the water (H2O) into hydrogen (H2) and oxygen (O) with the use of an electrolyzer.
If this electrolyzer is powered by renewable energy, for instance from solar panels or wind farms, the hydrogen produced has almost no carbon footprint. This ‘green hydrogen’ can then be used in many different applications.
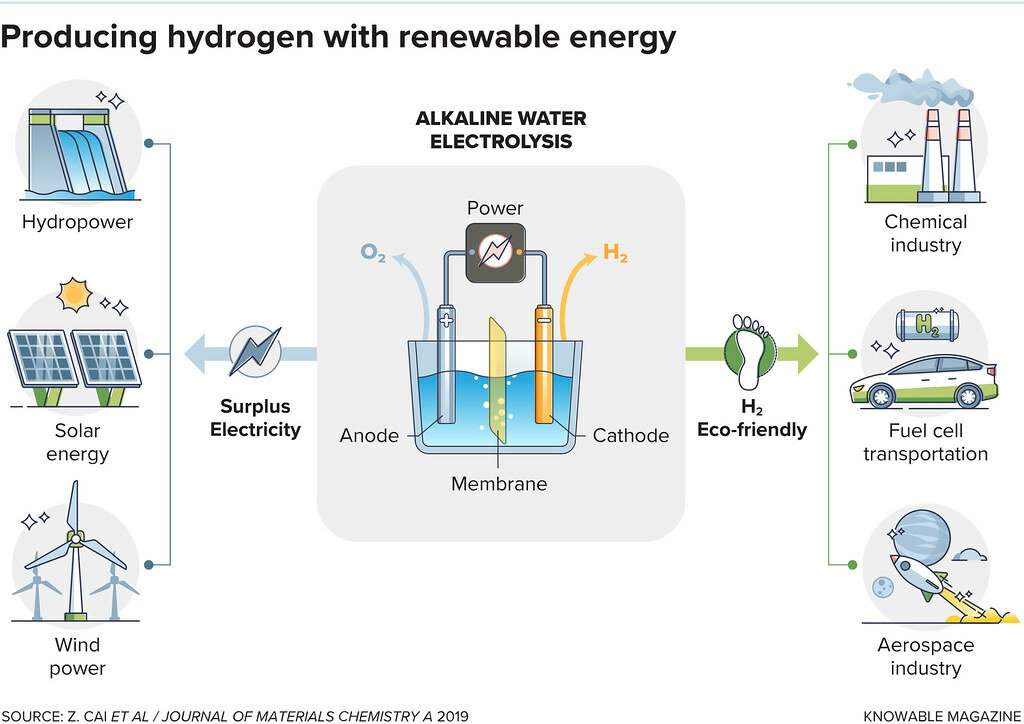
Electrolysis reaction:
2H2O → O2 + 2H2
What is the molar mass of hydrogen?
The molar mass of hydrogen gas (H2) is 2.01568 g/mole. This is because hydrogen gas is formed of 2 hydrogen atoms that each have a molar mass of 1.00784 g/mol. It can therefore be calculated by multiplying hydrogen’s atomic weight (1.00784 atomic mass units) by 2.
1.00784 x 2 = 2.01568 grams
How is hydrogen used in industries?
From airplanes to boats to drones, hydrogen is expected to play a leading role in many industries. But even today the gas is widely used for particular purposes:
- Hydro-desulfurisation (HDS) and hydrocracking operations: HDS is a process where sulphur is removed from natural gas and other petroleum products like gasoline, kerosene, diesel fuel and jet fuel.
- Ammonia production: The most common industrial process for the production of ammonia is currently the Haber-Bosch process. This involves nitrogen and hydrogen mixed at a 1:3 ratio, placed under pressure (150-250 bar) at a high temperature (400° to 650° C).
- Metallic ore reduction: Hydrogen can also be used to commercially extract tungsten from its ore (wolframite, scheelite, and ferberite). The same process can also be used to produce copper from tenorite and paramelaconite.
- Hydrochloric acid production: Producing hydrochloric acid (HCl) at scale has always involved producing other chemicals as by-products. Hydrogen can be combined with chlorine gas to produce hydrogen chloride in the presence of UV light. However this is rarely used in industry as it’s a very exothermic reaction.
- Hydrogenating agent: Hydrogen can be used to turn unsaturated fats into saturated oils and fats. This is particularly common in food industries, where hydrogen is used to create hydrogenated vegetable oils like butter and margarine.
What is “hydrogen electricity”?
Hydrogen electricity is electricity produced from hydrogen via a fuel cell. Hydrogen gas is fed into the fuel cells anode and air into cathode. A catalyst in the anode separates hydrogen molecules into protons and electrons, taking different paths to the cathode. The system passes the electrons through an external circuit, creating a flow of electricity.
It is this electricity that can be called “hydrogen electricity”.